News

New in Nature: Streamlining an A(H5) influenza vaccine
Antigenic cartography used to choose antigens for vaccine design
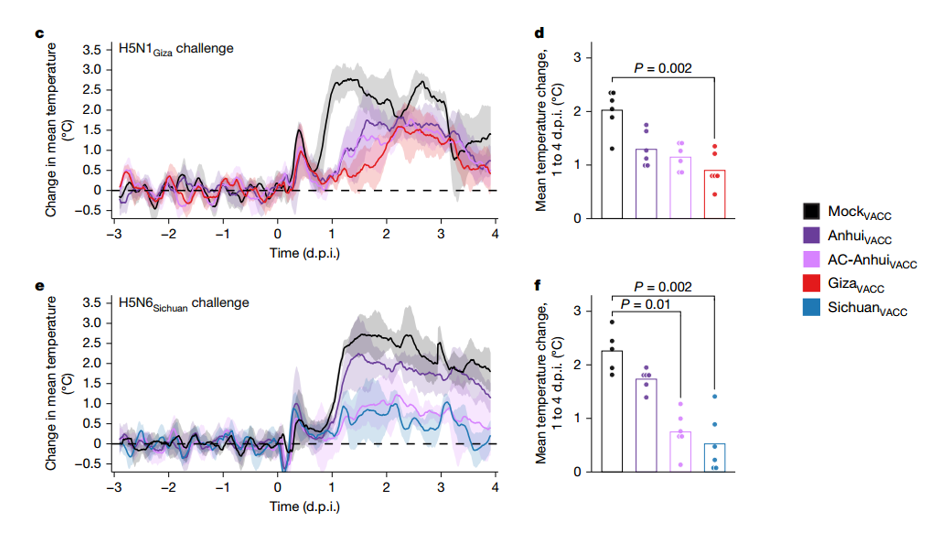
Hot off the press in Nature, a group of scientist in the Netherlands (Erasmus University and Utrecht University) and the UK (University of Cambridge) have mapped the global antigenic evolution of avian influenza A(H5) by constructing a high-resolution antigenic map of 127 HA antigens. They found out that the antigenic behaviour of the virus does not show the typical directional drift like H1 and H3 viruses but a non-directional structure. Using this map, they designed a centrally located antigen called AC-Anhui and tried it in a challenge experiment in ferrets using two types of strain from different clades: H5N1 Giza and H5N6 Sichuan, with homologous vaccines for those strains. Their central-antigen vaccine triggered antibodies that recognized a much broader range of H5 strains then conventional vaccines. It provided protection for both strains even if they were from different clades and the protection was comparable or superior to the strain-matched vaccines.
Star-Oddi loggers used in the challenge study
The group implanted 6 ferrets in each group with Star-Oddi DST micro-T temperature loggers 14 days after the prime vaccination. The loggers measured core body temperature every 10 minutes, which allowed for close monitoring of temperature changes throughout the challenge study as shown in figure 4 from the article. Temperature data from the loggers revealed reduced febrile responses in vaccinated groups, correlating with lower viral loads and milder clinical signs.
A potential model for future universal flu vaccine design
Although more work is warranted with follow-up studies in humans and other animals, the study introduces a new design principle for influenza vaccines where instead of chasing every new mutation, scientists can select an “antigenically central” strain that sit at the heart of subtype’s diversity. Such a vaccine could offer broad and durable protection that offering a major step towards pandemic preparedness for avian influenza.
The article was published in Nature and can be accessed here.photo from iStock