News
June 16. - 2023
Neutralizing Antibody Response Against Five Viruses Induced After Vaccination with MOPEVACNEW Vaccine
New World arenaviruses (NWAs) are known to cause haemorrhagic fevers and can have high mortality rates. Due to endemic NWAs viruses in South America and recent re-emergence of Machupo (MACV) virus, there is considerable need to discover novel approaches to vaccine development. Neutralizing antibodies (Abs) are critical in protection and control of NWAs. MOPEVAC vaccine, previously developed by the same group of scientists based on a hyper-attenuated Mopeia virus (MOPV), was shown to be efficient against the Lassa virus (LASV), an Old-World arenavirus, in macaques. It was therefore that a pentavalent MOPEVACNEW vaccine would provide protection against the deadly effects of all five NWAs know in South America. Should this vaccine prove to be efficient, it would be means of pre-emptive protection against outbreaks with known NWAs.
Body temperature measured for 96 days
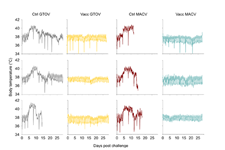
Scientists from the Pasteur Institute hosted at CIRI in France, implanted two groups of 12 female cynomolgus monkeys (CMs), Macaca fascicularis, intraperitoneally with Star-Oddi’s DST micro-T temperature loggers prior to the studies and were set to measure every 15 minutes. Temperature results from the Star-Oddi loggers can be seen below. The animals were immunized at day 0 and 56 and then transferred to BSL4 laboratory on day 89, where they were challenged with the viruses. Six animals were inoculated with each of the Machupo (MACV) and Guanarito (GTOV) viruses, that is three vaccinated and three unvaccinated animals.
No clinical disease detected in vaccinated animals
All the control, non-vaccinated animals showed signs of illness. In the MACV group the animals reached the ethical point of euthanasia between days 12-18 after exposure, and in the GTOV two days later. The course of disease was similar in both experiments. Signs of infection were found in organs and fluids of both groups and rise in body temperature is clear in the control groups. None of the vaccinated CMs developed clinical signs from which it can be concluded that it produces sterile protection (no virus detected in tissue or fluids) against the MAC and GTOV viruses.
Body temperature measured for 96 days
Scientists from the Pasteur Institute hosted at CIRI in France, implanted two groups of 12 female cynomolgus monkeys (CMs), Macaca fascicularis, intraperitoneally with Star-Oddi’s DST micro-T temperature loggers prior to the studies and were set to measure every 15 minutes. Temperature results from the Star-Oddi loggers can be seen below. The animals were immunized at day 0 and 56 and then transferred to BSL4 laboratory on day 89, where they were challenged with the viruses. Six animals were inoculated with each of the Machupo (MACV) and Guanarito (GTOV) viruses, that is three vaccinated and three unvaccinated animals.
No clinical disease detected in vaccinated animals
All the control, non-vaccinated animals showed signs of illness. In the MACV group the animals reached the ethical point of euthanasia between days 12-18 after exposure, and in the GTOV two days later. The course of disease was similar in both experiments. Signs of infection were found in organs and fluids of both groups and rise in body temperature is clear in the control groups. None of the vaccinated CMs developed clinical signs from which it can be concluded that it produces sterile protection (no virus detected in tissue or fluids) against the MAC and GTOV viruses.
Protection against all NWAs induced with MOPEVACNEW vaccine
Due to the severity of the disease produced by the NWA viruses the race is on to develop vaccine. The Pasteur team was able to develop and test a vaccine in a non-humane primate that protects against two distant viruses while also inducing neutralizing Abs against all pathogenic South American NWAs.
The article was published in Nature Biology and can be accessed here.
The article was published in Nature Biology and can be accessed here.